MSc BIT BSc HND Edxcel BCS ACS Programming Classes
GCE A/L O/L ICT Local London Cambridge Classes
Assignments & Final Year Project Help & Guidance
Website Development
Individual / Group / Online classes in English / Sinhala / Tamil.
Sample Projects/Assignments Exam Papers, Tutorials, Notes and Answers will be provided.
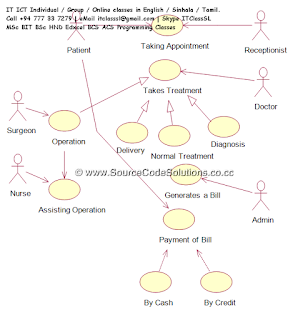
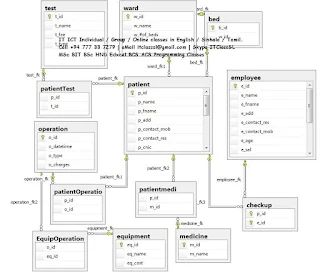
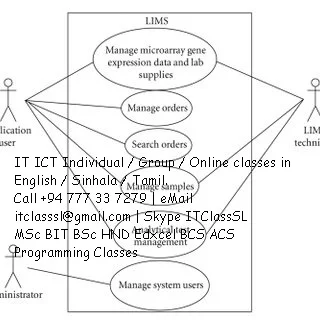
Laboratory management
system
A laboratory information management system (LIMS), sometimes
referred to as a laboratory information system (LIS) or laboratory management
system (LMS), is a software-based solution with features that support a modern
laboratory's operations. Key features include—but are not limited to—workflow
and data tracking support, flexible architecture, and data exchange interfaces,
which fully "support its use in regulated environments". The features
and uses of a LIMS have evolved over the years from simple sample tracking to
an enterprise resource planning tool that manages multiple aspects of
laboratory informatics. [https://en.wikipedia.org/wiki/Laboratory_information_management_system]
Key Benefits of a
LIMS
Although the primary purpose of a LIMS is to track and
manage samples, it can do so much more. It can:
·
Enable workflow automation which can in turn
reduce human error
·
Centralize access and storage of quality control
data
·
Support compliance efforts
·
Track reagents and lots
·
Perform instrument run monitoring
·
Initiate downstream data analysis
·
Integrate with instruments or other in-lab
systems to improve lab efficiency
[https://www.illumina.com/informatics/sample-experiment-management/lims.html]
Detailed
Functionality of Our LIMS Includes:
·
User definable sample labels with the capacity
to have the labels pre-printed
·
Login-sheets with bar-codes, supporting most
bar-code layouts.
·
Receipt of Samples
·
Registering of samples into the Labmin
Laboratory Information Management System
·
Unique name allocations to individual samples.
·
Sample numbers, prep batch and quality control
of batch numbers. Both at the Laboratory as well as online.
Integration of the Labmin Laboratory
Information Management System with Analytical Instruments and Various
Applications
·
Allows created analytical data to be stored
directly into the Labmin Laboratory Information Management System; eliminating
any typographical and transcription errors.
·
Document control and data protection tools to
manage procedures and important files.
·
Unique identity of instruments with respect to
calibration status and service maintenance.
Integration of LIMS to External Systems
·
Accounting packages e.g. PASTEL.
Please request if you want to know who we have successfully integrated with.note
·
Creation of certificates of completed analysis
to internal customers like geologists to easily retrieve the results. These are
customised as per your client’s needs.
·
Warehouse Module for control of all consumables.
·
A BI (Business Intelligence) module is also
available on request.
·
Multiple Language Interfaces allows the client
to receive reports in their preferred language.
Billing / Pricing & Invoicing
·
Intergration of Automatic billing of completed
projects for clients
·
Integration your specific ERP
requirements/system.
·
Create and manage quotations for laboratory.
·
Provide different pricing for individual
clients.
·
Allows management of your implemented processes.
·
Physical tests can be set up at four different
levels and priced accordingly. Pricing of tests helps you generate accurate
invoices to decrease financial losses and discrepancies.
·
Tracks the profitability of each client
·
Allows you to track which customers are more
profitable and how frequently they make use of your service.
·
Discount allocation per job/client
·
Tracking test results per client
·
Delivery of detailed trends analyses per
customer
·
Delivery of detailed sample results per customer
·
Multiple contacts per client
·
Debtor Management
·
Entry of single or multiple samples.
·
Automatically assigns sample numbers.
·
Automatic calculations for due and hold dates,
both for preparation and for analysts.
·
Sample and test copying of common samples.
·
Options to put timers on individual samples.
Laboratory work flow management
·
Grouping of samples into work lists, preparation
phases, and QA/QC batches.
·
Reviewing, printing and exporting of sample
statuses and allows clients to login and add comments.
·
Warnings for samples nearing hold or due dates
in the form of alarms, pop-up messages etc.
·
Links between sample status and result.
·
Additional laboratories can be added or deleted
from Labmin.
·
These laboratories can be managed and viewed
individually.
·
Advanced reporting per laboratory measures
profitability and effectiveness.
·
Problem or excelling areas can be identified and
managed for profitability and optimisation in real time.
·
Record the whereabouts of samples and their
movement within and between the laboratories.
·
Sample backlog tracking.
·
Automatic generation of work orders and sample
numbers with user definable formats.
·
Archiving samples from an active database.
·
Automatic printing of samples for disposal.
·
Entry by test, sample number, work list, or
other information from Sample Master’s “Master Query”.
·
BI Tool that allows more sample information.
Approval and Review Management
·
Used for implementation of new lab methods.
·
Traceability of raw data as first generated by
instrument or analysts i.e. raw data transferred to the Labmin Laboratory
Information Management System.
·
Analyst training, competency records, SOP’s and
material safety data sheets (MSDS).
·
Automatic limit checking of results, when within
limits. Automatically authorize reject or re-test, when not.
·
Automatic control chart checking on entry of
QA/QC results.
·
Direct export of results to spreadsheet or other
file formats.
·
Separate entry, validation and approval of
results for reporting.
·
Generation of control charts by analyst,
instruments, date, range etc.
·
Automatic accuracy and precision calculations
when all formulas for calibrations are added into the solution.
·
Stock Control reports with QA/QC information as
part of the analytical report.
·
Inventory management of standards and reagents,
certificates of analysis and automatic expiry notifications.
·
Frequency based scheduling for automatic login,
including workload analysis for upcoming samples.
·
Direct importing from spreadsheet with “point
& click” file selection.
·
Storage location tracking e.g. water samples and
chain of custody for each sample.
·
Define user capabilities/access and enables
restriction protocols by user roles:
o
LIMS administrator
o
Laboratory Manager
o
Quality Manager
o
Lab Supervisor
o
Analyst
o
Customer etc.
·
An unlimited number of user definable custom
reports.
·
Functions for internal clients to call-up and
retrieve only their sample information.
·
Direct printing of reports to user’s printer and
emails.
·
Keeps records of trained and certified analysts
able to run the analysis as well as certifying of other analysts (competency
records)
·
Automatic production of test report/certificate
as per clause 5.10 of ISO 17025.
·
Availability of data supporting the reliability
of the information on test report (vertical auditing).
·
Audit trail reports.
·
Management reports.
·
End-user reports.
·
Statistical quality control for instrument
calibration data, ensuring optimal equipment status.
·
Secure reporting and electronic signatures.
·
Electronic worksheet for producing results
e.g. standards and reagents preparation, instrument calibration, review and
revalidation details, sample batches, environmental and preparational summary,
calculation worksheets etc.
·
Report review facility
Proficiency Testing Schemes and
Inter-Laboratory scheme
·
Management of results for such
schemes/studies
Only if added into the system as an additional solution.
·
Comprehensive training for laboratory personnel
with different roles required for the Laboratory Information Management System.
·
LIMS Administrator training.
·
End user training and documentation.
·
After Sales Support/Technical Support.
·
Onsite Support (Travel and Accommodation not
included).
·
The solution also caters for Satellite based
laboratories to log into Labmin for the test & it’s results to be uploaded
in real-time.
Giving your laboratory a much bigger scope to work with.
http://labmin.com/lims-overview/lims-features/
Laboratory execution
[https://www.simplelimssoftware.com/limsfeatures.html]
The Simple LIMS software is further
configurable by users and system administrators to quickly match changing
needs, protocols, business rules, workflows, etc., without coding. More detail...
·
Configure
testing workflow, data model and business logic without programming.
·
Define/configurator lab testing workflows, feature include: Workflow
attribute definition of activities, workflow input and output activities
·
Assignment of documentation to activities
·
Dynamically modify workflow in case of future change and assignment of
documentation to activities
·
Assign
work to scientists (task owner, and approved by), transfer the workflow
information to Electronic Data Exchange format (export to PDF or Excel format)
and attach the SOP documents with every workflow
·
Manage
and analyze workflow testing results with accurate range
·
Define
a group of sample batches and manage the workflow batch information
·
Manage
workflow activities history records
·
Lab
manager workflow define approved process by e-signature.
Samples, sample inventory management
·
Storing
related sample information
·
Tracking samples by sample ID and barcode
·
Track
samples from the time they enter the lab, to the time they are completed and
disposed. Generate a Chain of Custody (COC) report.
·
Sample
instant information management, define sample instant and series.
·
Sample
inventory plate and location information management and sample destroy schedule
management
·
Create
data report association for samples
Lab customer's order/case management
·
Generate customer orders
·
Case
accessioning and assignment
·
Manage
order's project and task information, including job allocation, instrument
allocation
·
Monitoring
project/test workflow's progress status and generate order result reports
·
Generate order resource allocation report (include order, project,
workflow, lab reagents using amount, instruments, history records and man power
usage) and document and result association
Customers information management
·
Customer information management
·
Customer testing result analysis and management
·
Customer billing and sample inventory control.
·
Send
email to customer for testing results and invoice.
Lab reagents inventory management
·
Lab
supply & reagent inventory management, reagents expiration date alerts
·
Lab
supply and reagents purchase order and payment schedule management
·
Track
inventory reduction based on usage, automatic decrease the reagents quantity
after each project/order done
·
Laboratory material vendor's information management
Instruments management
·
Lab
instruments information management, instrument's tests assigned to and access
records management
·
Instruments maintenance record and maintenance due date alert
·
Instrument maintenance records table management
·
Track
location and usage of laboratory equipment
Instrument data input
·
Instrument
data input through RS 232/USB/Windows Serial Port by following MS Windows
standard drive interface
·
Open
source solution allow all our customers modify/update current tables and forms
and link with instrument input
·
Input
spreadsheet, text file html file data by following MS Access data input process
·
Instrument input/output documents file information management
Laboratory administration management
· Company and employee information management,
includes employee training records attachment file for ISO900 checkup and
employee workload reports
· Dashboards view all your performance
indicators on main administration page; keeps track of equipment's maintenance,
shelf life and other sensitive item alerts
· Generate company accounting receivable and
payable schedule information list forms for you to review the cash flow.
· Generate batch and project due today reminder
for lab operating process
Data retrieval and document management
· Manages workflow, tasting and projects
documents information and exports to PDF or Excel format for Electronics Data
Exchange
· Manages SOP and other documents for lab's
tracking needs
· Standard operation process for documents and
report information management. Feature include documents name, description,
file date, type, and categories. you can trace document's information by using
these information and open the attachment file by simple click.
· Generate industry standard reports
· Define a data query using search filters and
export data.
· Export to PDF or MS Excel format
· Add documents as attachments
Billing information management
· Generate order billing invoice and sample
testing result for each project
· Expense reporting
· Revenue management
· Bill of materials
· Grant management
Login/Accession
· Users login with default 3 different levels
(Admin/Manager, lab employee and lab receptionise)
· Users can login by user name and password
with different access and control of this LIMS software
· User can modify the login method based on
their lab process needs.
LIMS Features &
Functionality
·
Lab Inventory & Storage Management
Know where everything has been and
for how long with sample and container tracking, location audit logs, etc.
Manage stock supplies and reagents; assign re-order alerts.
·
Lab Project Management
Create and manage lab project
workflows of samples through assays, standardize methods and procedures.
·
Electronic Document Management
Associate any electronic file to
any object within the LIMS using links. From raw data to final study reports,
the Core LIMS is a unified repository for all of the documents required to
support a lab’s research.
·
Laboratory Automation & Integration
Instrument control for liquid
handling and synthesis work stations including Tecan Genesis, Cyclone, Tomtec,
etc.; integration with third party systems (ERP/PIMS etc.).
·
Automated Data Capture & Reduction
Direct integration with data
generating instruments, custom file parsers and data loaders, and automatic
data reduction.
·
Chemistry & Cheminformatics
State of the art cheminformatics
platform including: compound registration, structure visualization, drawing and
searching, calculated properties, etc.
·
Molecular Biology, Cell Culture &
Bioinformatics
State of the art bioinformatics
platform providing a variety of functionality, including integration with
instrumentation.
·
High Throughput Screening (HTS)
Automates the collection and
validation data, generates automatic alerts. Allows for in-vitro data
management.
·
Animal Study Management
Tracks animal study design and
manages workflow for PK, efficacy, behavioral, and toxicological studies.
·
Animal Facility Management
Cage card printing, animal
location management and history, comprehensive record tracking, and IACUC
protocol management and enforcement.
·
Customized Reporting
Define custom report formats for
any data type. Automatically run reports, export results to MS Word,
PowerPoint, Excel, and Adobe PDF.
·
Laboratory SOP Enforcement
Enforce business rules directly
within the system before a sample is registered, screened or queued.
·
Workflow Management
Implement project decision
criteria to ensure samples of interest are automatically queued for screening.
Capture data for audits and compliance with regulatory standards.
·
Prioritization Queues & Worklist Management
Assess current workload and
re-assign priorities for each assay. Identify bottlenecks in project
lifecycles.
·
Instrument Management
Monitor the status of the lab’s
instruments, schedule work and maintenance.
·
Security
Control access to data, track all
changes made in the system to documents, data, etc.
·
Environmental Monitoring
Monitor conditions in the
laboratory and storage spaces. Control waste generation and disposal.
·
Lot, Batch, & Case Management
Manage lots, batches and cases as
needed. Increase efficiency with grouped sample login etc.
·
Specifications Management
Manage customer specifications and
custom recipes. Regulatory compliance.
·
Data Analysis
QA/QC, graphing, curve-fitting,
NCA, calculations, etc.
·
Study Management
Create custom and reusable study
designs, report data by study, results validation and management.